Projects
In blood, complement proteins are a major component and perhaps therefore most of these were identified already decades ago. However, only in recent years it has become apparent that complement not only plays a major role in innate defense against pathogens but also identifies foreign materials and removes waste (immune complexes and dying cells). The physiological relevance of complement is demonstrated by diseases affecting patients lacking complement components: recurrent infections, autoimmune diseases and glomerulonephritis.
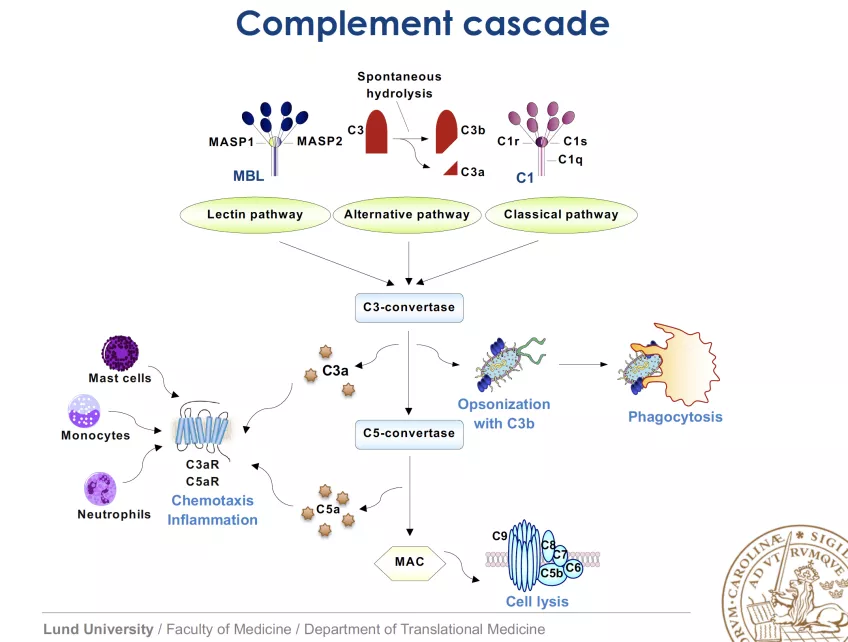
Regrettably, uncontrolled complement activation also contributes significantly to pathology of many diseases (some examples: rheumatoid arthritis, ischemia/reperfusion injury, glomerulonephritis, multiple sclerosis, Alzheimer´s, hyperacute rejection of grafts) due to the fact that complement sometimes misdirects its activities towards own tissues. Invading pathogens activate complement either spontaneously due to differences in envelope/membrane composition compared to host (alternative and lectin pathways) or through antibody binding (classical pathway). This leads to initiation of cascade of enzymatic cleavages and formation of crucial enzymatic complexes (C3 and C5 convertases), release of pro-inflammatory anaphylatoxins (C5a, C3a) that attract white blood cells and finally formation of membrane attack complex (MAC, pore in a membrane).
Considering the destructive potential of the complement system, it is no surprise that nearly half of the system’s proteins are involved in its inhibition. Several of these inhibitors circulate in blood whereas others are expressed on virtually all cells of the body to protect self-tissue from complement attack. C4b-binding protein (C4BP) is the major soluble inhibitor of the classical and lectin pathways whereas factor H (FH) inhibits the alternative route. Most inhibitors act on complement convertases through increased dissociation of these enzymatic complexes (acceleration of decay) or through promoting enzymatic cleavage of activated complement factors C3b or C4b by a serine proteinase factor I (FI). Some microorganisms either produce a functional mimic of a complement regulatory protein or hijack host’s regulatory proteins. This allows them to circumvent adversary effects of recognition by complement.
Our group investigates the physiological regulation of human complement system as well as pathologic situations when this regulation fails.